Alkali-Activated Metakaolin
Due to the abundant availability of kaolin clay around the world, alkali-activated metakaolin (AAMK) (a.k.a. alkali-activated calcined clay) has the potential to significantly lower CO2 emissions associated with the cement industry (reduction of 65% or more). Metakaolin is manufactured by calcining (i.e., heating) kaolin clay at 700-900°C. Worldwide reserves of kaolin clay are thought to be greater than that of limestone, making metakaolin well-suited for use by the concrete industry.
There are several challenges that need to be overcome to increase the likelihood of AAMK concrete becoming a widespread commercial reality, some of which are the focus of our research:
- Reducing the alkali demand required for activation (CO2 and cost savings)
- Reducing the water demand and achieving optimal rheology
Our recent work in Cement and Concrete Research has shown that replacing 10 wt.% of the metakaolin with an earth alkali (such as calcium hydroxide) can be used to increase the reaction kinetics of reduced alkali AAMK mixes.
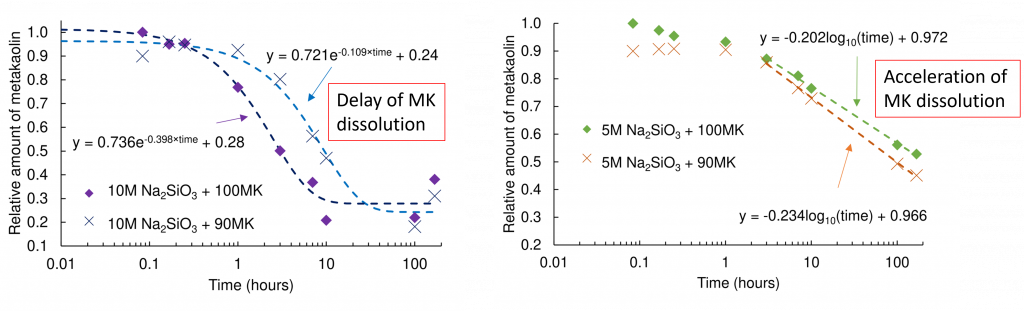
This replacement in reduced alkali AAMK mixes also improves the durability via refinement of the pore structure, as outlined in another of our recent work in Cement and Concrete Research.
Carbonate Materials
Carbon sequestration is an area of research to address the increasing amounts of CO2 being released into the atmosphere. One aspect of this technology involves injecting CO2 into depleted geological formations so that the CO2 molecules react with existing phases to form stable mineral compounds. Other technologies include sequestering CO2 above ground, such as via minerals carbonation. Therefore, understanding materials containing carbonate phases (various forms of CO3) is of relevance for carbon storage, including cementitious materials that come in contact with CO2 during the sequestration stage. SCG is investigating the disordered carbonate phases that can develop in alkali-activated materials when exposed to elevated CO2 levels, and has shown that the development of ‘stable’ amorphous carbonates is linked with a lower extent of binder (cement) degradation.